Poster presented at American College of Neuropsychopharmacology in December 2023.
Conclusions:
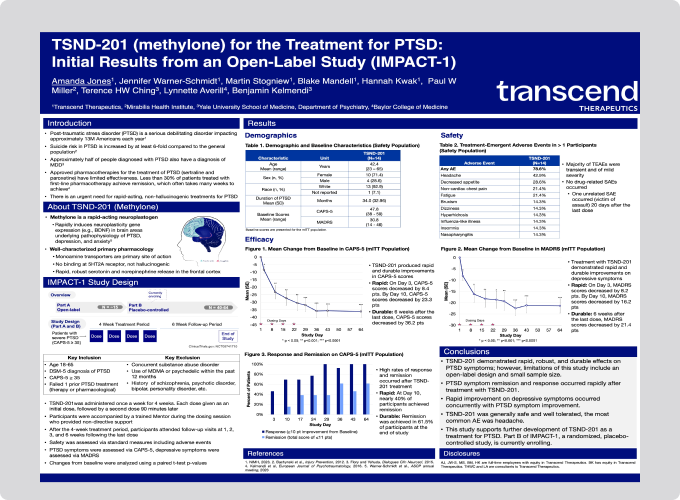
- TSND-201 demonstrated rapid, robust, and durable effects on PTSD symptoms; however, limitations of this study include an open-label design and small sample size.
- PTSD symptom remission and response occurred rapidly after treatment with TSND-201.
- Rapid improvement on depressive symptoms occurred concurrently with PTSD symptom improvement.
- TSND-201 was generally safe and well tolerated, the most common AE was headache.
- This study supports further development of TSND-201 as a treatment for PTSD. Part B of IMPACT-1, a randomized, placebo-controlled study, is currently enrolling.
)



